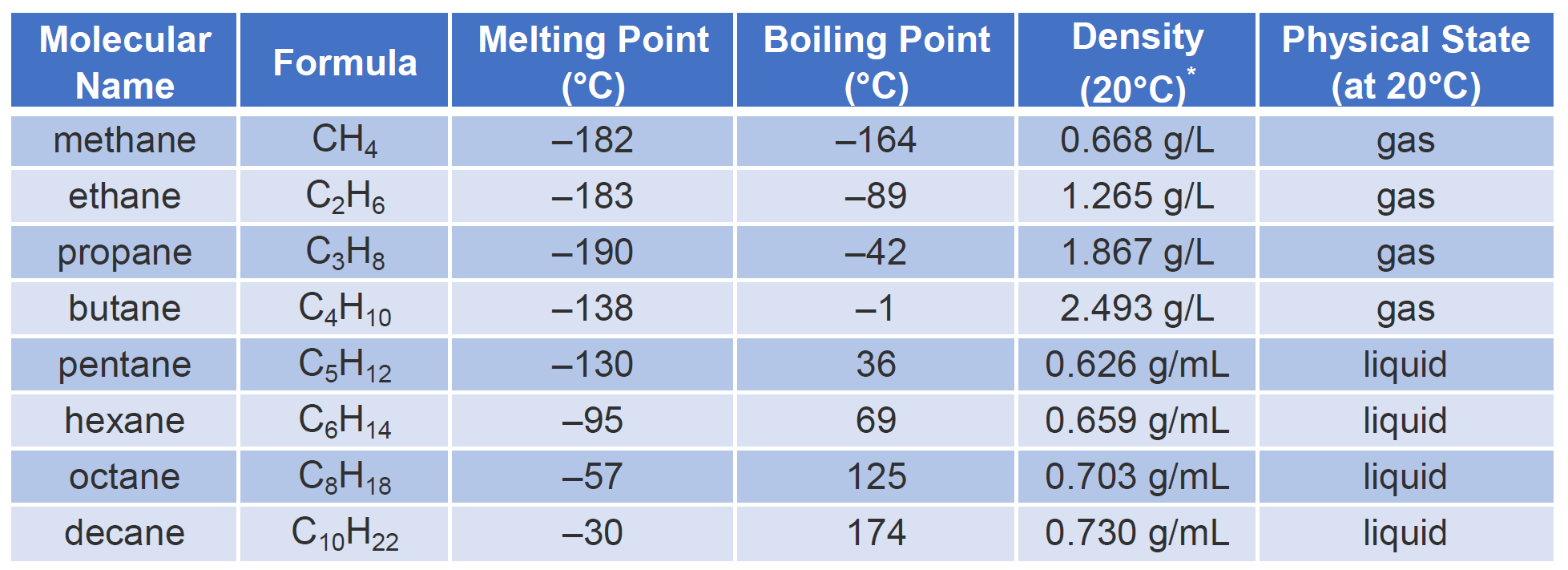
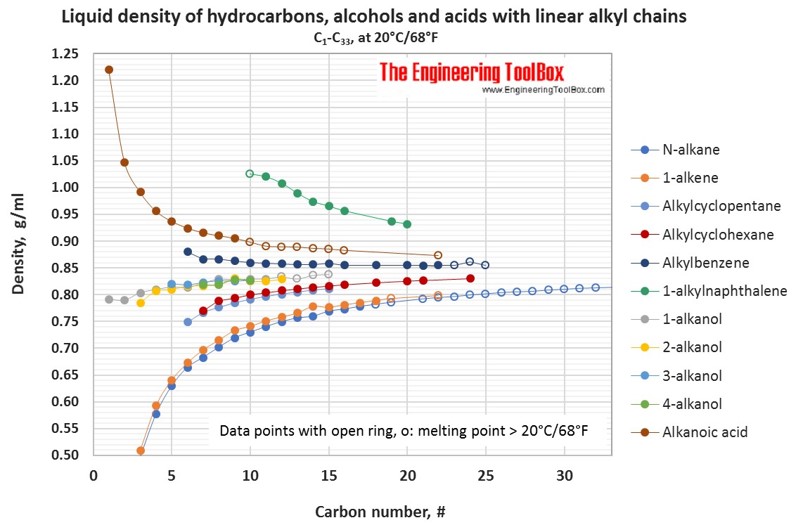
The densities of alkanes Table 21 increase with increasing molecular weight but even a 30- carbon alkane such as tria-contane density at 20 C 08097 gmL is less dense than water density at 20 C 09982 gmL. However gasoline has an odor and some color because dyes are added to gasoline by refiners to.

8 13 Physical Properties Of Hydrocarbons Chemistry Libretexts
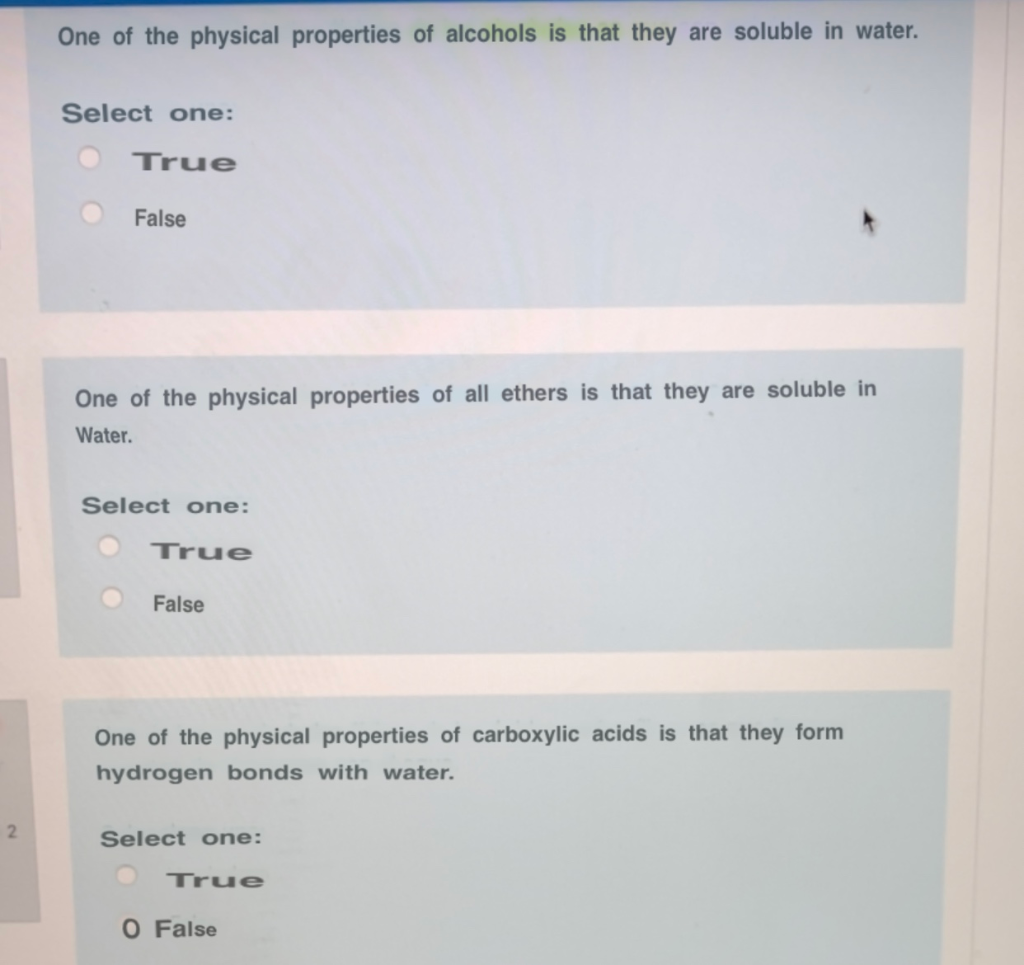
The carbon-halogen bond is more polar than the carbon-hydrogen bonds but most alkyl halides are not very soluble in water.

. Name the difference between a saturated and an unsaturated hydrocarbon. These properties explain why oil and grease do not mix with water but. Alkenes are more reactive than their related alkanes due to.
Melting Point and Boiling Point of Alkanes. The densities of Alkanes are lower than the density of water. Therefore the prior is less denser.
Alkanes are in water and than water. Alkanes are _____ in water and _____ than water. True or False the two main sources of alkanes in the world are petroleum and natural gas.
Their density value is nearly 07 g mL-1 considering the density of water as 10 g mL-1. Alkanes are less dense than water alkanes will float on top of water Density increases with increasing molar mass. Nearly all alkanes have densities less than 10 gmL and are therefore less dense than water the density of H 2 O is 100 gmL at 20C.
Zinc is more dense than water. This means that a mixture of an alkane and water will separate into two distinct layers with the less dense alkane. Complete combustion of pentane produces.
Saturated hydrocarbons do not contain ultiple bonds between carbons but unsaturated hydrocarbons do contain multiple bonds. Like pentane quite a large mass number. Thus gasoline which is largely a mixture of alkanes is less dense than water and will float on water.
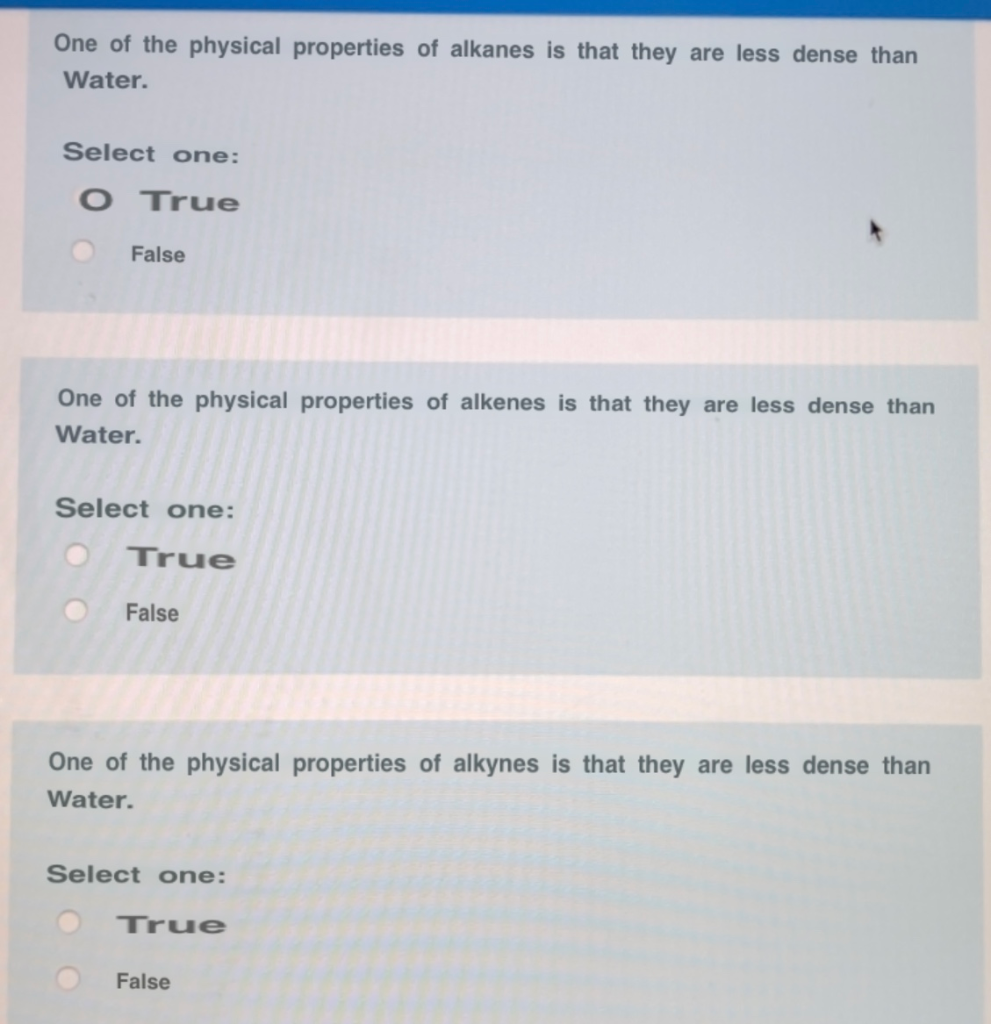
Consequently alkanes themselves are commonly used as solvents for organic substances of low polarity such as fats oils and waxes. False all alkanes are less dense than water. Is zinc less or more dense than water.
They are generally soluble in organic solvents. Bearing in mind that only alkanes from Pentane C5H12 or higher molecular mass are liquids or solids above 0 C the solidification point of water In simple terms water is of course H2O with a. Alkyl fluorides and chlorides having only one halogen atoms have densities that are higher than those of alkanes but are slightly less dense than water while alkyl bromides and iodides are generally more dense than water.
Mostly because oxygen atom is heavier and also smaller than carbon. In addition they do not conduct electricity. Table 73 Physical Properties of Some Alkanes.
Alkenes are non-polar and they are both immiscible in water and less dense than water. These properties explain why oil and grease do not mix with water but. View the full answer.
Nearly all alkanes have densities less than 10 gmL and are therefore less dense than water the density of H 2 O is 100 gmL at 20C. For the simple alkanes composed of carbon and hydrogen yes. Methane to butane have boiling points less than 25C.
Insoluble more dense d. Why are most hydrocarbons like alkanes and alkenes less dense than water. Nearly all alkanes have densities less than 10 gmL and are therefore less dense than water the density of H 2 O is 100 gmL at 20C.
Alkanes have densities between 06 and 08 gcm 3 so they are less dense than water. Density of Alkanes. Show activity on this post.
Insoluble less dense soluble less dense O insoluble more dense a O soluble more dense Previous Page Next Page. Alkanes are insoluble in water and less dense than water. Pure alkanes are colorless tasteless and nearly odorless.
Soluble less dense c. Previous question Next question. Insoluble less dense b.
Answer 1 of 2. True or False All alkanes that are liquid at room temperature are more dense than water. Alkanes are non-polar Intermolecular forces are van der Waals forces Insoluble in water Less dense than water.
For example if we mix an Alkane with water the Alkane layer separates on the top of the water since Alkanes are less dense compared to water and they are insoluble in water. True or False there are four alkyl groups with the molecular formula C4H9. Consequently alkanes themselves are commonly used as solvents for organic substances of low polarity such as fats oils and waxes.
But hydrocarbons have so many carbon atoms. Question 13 1 point Alkanes are - in water and than water. Alkanes are generally unreactive toward laboratory acids bases oxidizing agents and reducing.
Carbon dioxide and water. Alkanes are gases where water is a liquid. Simple alkanes have low melting and boiling points measured at 1atm or 1013 kPa pressure.

Physical Properties Alkanes 2 Physical And Chemical Properties Covalent Bonding Physical Properties

Solved Question 24 1 Point Which Of The Following Is Are Chegg Com

Because Of Hydrogen Bonding Water Is Less Dense As A Solid Than It Is As A Liquid Consequently Ice Floats Chemistry Outline Biology

Solved Which Of The Following Is A General Physical Property Of Alkanes Low Solubility In Water More Dense Than Water Less Volatile Than Water More Polar Thanwater
Are Alkanes More Dense Than Water Quora
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry

Are All Alkanes Lighter Than Water Quora

Chapter 11 Introduction To Organic Chemistry Alkanes Ppt Video Online Download

Storehouse Of Frozen Methane May Be Future Energy Source Elephant Journal Beautiful Nature Abraham Lake Beautiful Places
Solved One Of The Physical Properties Of Alkanes Is That Chegg Com
Hydrocarbons Linear Alcohols And Acids Densities
Solved One Of The Physical Properties Of Alkanes Is That Chegg Com
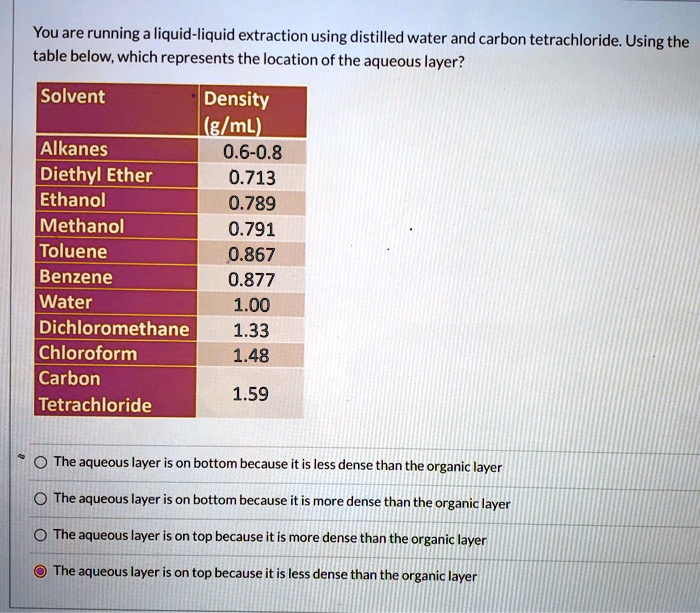
Solved Youare Running A Liquid Liquid Extraction Using Distilled Water And Carbon Tetrachloride Using The Table Below Which Represents The Location Of The Aqueous Layer Solvent Density I Elml Alkanes 0 6 0 8 Diethyl Ether 0 713 Ethanol 0 789

Solved Compare The Densities Of Liquid Alkanes With That Of Water If You Added Octane To Water In A Beaker What Would You Expect To Observe

Properties Of Alkanes Nonpolar Molecules Not Water Soluble Relatively Low Melting And Boiling Points Generally Less Dense Than Water The Longer The Chain Ppt Download
Why Is Ether Less Dense Than Water Quora

Solved Which Of The Following Is A General Physical Property Chegg Com

Solved What Generalizations Can You Make About The Densities Of Alkanes Relative To The Density Of Water

